Key Takeaways
- Exploring the multifaceted nature of ethics in clinical trials.
- Recognizing the significance of informed consent for participant autonomy.
- Examining the balance between the pursuit of scientific knowledge and participant safety.
- Understanding the role of regulatory frameworks in maintaining ethical standards.
- Appreciating the importance of privacy and data protection in clinical research.
- Understanding how ethical solid practices underpin public trust in clinical research.
- Considering the global implications and challenges of clinical trial ethics.
- Contemplating the evolving ethical landscape in response to new technologies and scientific advancements.
The Foundation of Ethical Clinical Trials
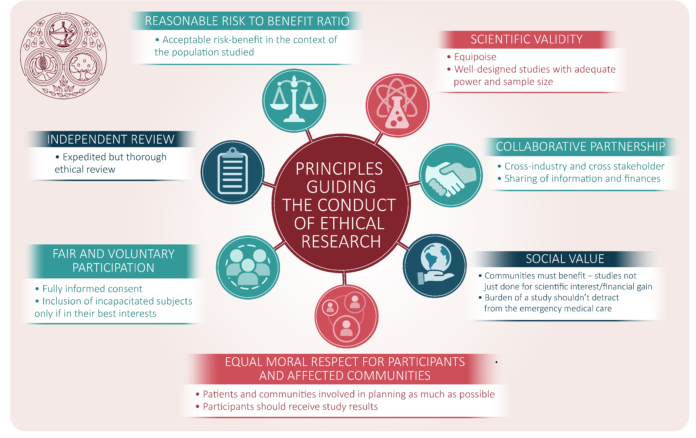
As the principal means of evaluating novel therapies and pharmaceuticals, clinical research provides the foundation of healthcare innovations. The ethical standards for these trials ensure that they are scientifically sound and morally responsible. A thorough assessment of the benefits and risks of clinical trials is fundamental to this process, securing the well-being of participants as the highest priority. Exacting ethical scrutiny is not an obstacle to progress but a guarantor of meaningful and trustworthy research outcomes.
The emphasis on an ethical framework within clinical research is essential to balance the urgency of developing new therapies and safeguarding participants’ rights and safety. By meticulously considering the implications of each research decision, the integrity and ethical rigor of clinical trials can be upheld, thereby supporting the health of individuals and the community.
The Role of Informed Consent
The practice of informed consent is a pivotal aspect of ethical clinical trials, rooted in the fundamental belief in an individual’s right to self-determination. Participants are thoroughly informed on the trial’s purpose, scope, and possible outcomes. Accurate details about the processes, potential risks, anticipated advantages, and substitute therapy options are also provided. This transparent communication ensures that individuals make a well-considered decision to participate, fostering an atmosphere of trust and openness between researchers and participants.
Despite its complexity, the informed consent process goes beyond mere regulatory compliance; it reflects the research community’s respect for the ethical principle of autonomy. Since this is the first step towards developing a positive connection between the research team and the participants built on mutual respect and trust, it must be completed with the utmost care and honesty.
Balancing Science and Safety
Pursuing scientific discovery through clinical research must always be counterbalanced by uncompromising attention to participant safety. It entails a continuous risk assessment and adaptation process to ensure the highest possible safety standards. Researchers collaborate with medical ethicists, patient advocacy groups, and oversight committees to examine and mitigate any ethical concerns that arise during the trial’s progression.
Safety measures are not static; they evolve in tandem with the trial’s phases and as the understanding of the intervention deepens. Protocols for monitoring participant health and responding to adverse events are rigorously defined and adhered to preserve the welfare of every individual involved in the trial. Flexibility in protocol and an unwavering commitment to safety are critical for the ethical conduct of clinical research.
Regulatory Frameworks and Oversight
Regulatory frameworks are:
- The backbone of ethics in clinical research.
- Comprising laws.
- Guidelines.
- Institutional measures are designed to standardize the ethical conduct of trials.
These frameworks encompass many stipulations, from establishing Institutional Review Boards (IRBs) to adhering to international declarations such as the Helsinki Declaration that set ethical standards across countries. These regulations are constantly refined to respond to emerging ethical dilemmas and accommodate the rapidly evolving medical research landscape.
Compliance with these frameworks is meticulously monitored to ensure that each clinical study is executed according to ethical best practices. The entire process, from the pre-trial phase to the publication and dissemination of results, is subject to comprehensive evaluations and audits to confirm adherence to ethical principles. This level of oversight is crucial for maintaining public confidence in clinical trials and the institutions that conduct them.
Privacy Concerns in Clinical Research
The sanctity of participant privacy is an ethical imperative in clinical trials. Strict privacy policies protect personal and health information throughout the trial and even after its conclusion. Scholars are highly concerned about safeguarding personal information, as seen by their compliance with US laws such as the Health Insurance Portability and Accountability Act (HIPAA).
Technological innovations, such as encrypted databases, authentication protocols, and anonymization techniques, are continually being developed and implemented to bolster participant data security. These efforts serve the interests of individuals and fortify the integrity of the research by preserving the trust essential for the continuation of valuable scientific inquiry.
The Impact of Ethics on Public Trust
Public trust in clinical research is fragile and heavily dependent on researchers’ visible commitment to ethical conduct. Essential to this is meaningful engagement with the public, where researchers uphold transparency and allow scrutiny. Researchers nurture an environment of credibility by clearly communicating the objectives, methods, and findings of trials and acknowledging and addressing ethical concerns.
This commitment to ethical practices goes a long way in solidifying the faith that participants and the public place in clinical trials. It also encourages the continued voluntary involvement of individuals in future research, which is indispensable for the ongoing pursuit of medical breakthroughs. Establishing straightforward reporting mechanisms and making results accessible are further practices that convey respect for the contributions of participants and the importance placed on ethical standards.
Global Perspectives on Clinical Trial Ethics
As clinical trials become increasingly global, the ethical considerations become more complex. The international character of many studies requires an adaptable ethical framework that respects local customs and regulations while upholding universal moral standards that transcend geographic and cultural boundaries. Researchers must exercise cultural sensitivity and consider local ethical perspectives without compromising on core principles fundamental to ethical research.
Such challenges have led to collaborative platforms where ethicists, researchers, and regulators from various countries work together to harmonize ethical guidelines for international trials. There must be a vigorous global conversation to guarantee that participant rights and welfare are upheld globally and that the outcomes of multinational trials are reliable and morally sound.
Future Challenges in Clinical Trial Ethics
The fast-paced evolution of science and technology presents ongoing challenges for clinical ethics. Innovations such as personalized medicine, gene therapy, and artificial intelligence introduce novel ethical questions requiring vigilant and proactive approaches. As the complexity of these issues grows, the ethical frameworks guiding clinical trials must adapt accordingly.
Anticipating future ethical dilemmas and cultivating a proactive discourse around ethical considerations in clinical research is crucial for responsibly preparing the scientific community to navigate these uncharted territories. The principles of safeguarding participant welfare, respecting autonomy, and ensuring transparency remain as relevant as ever as we march forward into the future of medical science.
Suggested reads: The Untold Story of Finlo Clarkson: Car Accident, Siblings, and What Happened to Him